Trapping a Bacterium in a Laser Beam Aids Study of Biofilms
Biofilms are responsible for most chronic infections and are notoriously resilient and hard to treat.
Scientists at The University of Texas at Austin have developed a technique to move and position a single bacterium using a highly focused laser. The precise control offered by this tool will allow researchers to better study how bacterial biofilms form.
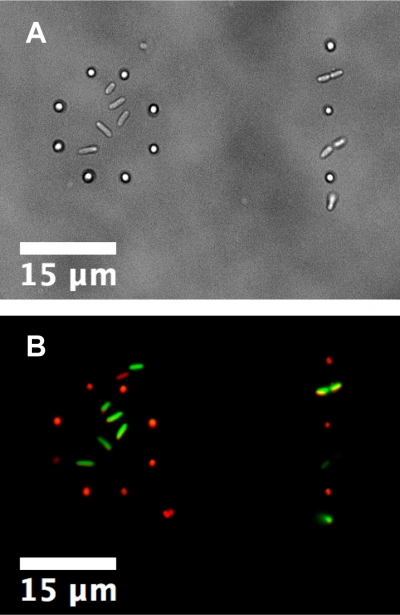
The use of a laser trap allows for precise placement of bacteria for studying biofilm development. Here cells of two different species, Pseudomonas aeruginosa and Staphylococcus aureus, were both trapped and placed in mixed-species patterns. On the left a ring of S. aureus encircles a small cluster of P. aeruginosa and on the right is an alternating line of each species.
A) Bright-field image of the initial placement.
B) Two-channel fluorescence image of the same area with P. aeruginosa in green and S. aureusin red. Images are from the study published in Langmuir.
Biofilms are responsible for most chronic infections and are notoriously resilient and hard to treat. In U.S. hospitals the films can grow on catheters and implanted devices and cost billions of dollars annually to treat. A better understanding of how these pathogenic films form and develop will aid in their prevention and treatment.
Although bacteria are typically thought of as moving around their environments without restriction, the pathogenicity of many species of microbes greatly increases when the organisms hunker down and stay put, forming what is known as a biofilm. The bacteria embed themselves and the surface beneath them in a sticky slime, resulting in a community that is resistant to cleaning agents and antibiotics and does more damage to host tissues.
It is widely believed that the structure of the biofilm is important for its disease-related properties. The spacing and location of each bacterium with respect to its neighbors, as well as the variety of bacteria present, can all affect the formation, strength, and biological activity of a biofilm. However, we know very few specifics of how this works, in terms of specific spatial structures and combinations of multiple microbial species.
Unfortunately, most existing methods for studying bacteria and biofilms cannot efficiently examine the effects of specific types of spatial structure. Furthermore, the presence in biofilms of multiple species of microbes is often not taken into account. Those methods that do use multiple strains often work with bacteria that are free-living, not in biofilms.
A new method developed by UT researchers will contribute toward changing that.
“What we’ve shown is that we can construct a biofilm that has a structure we impose on it at the single-cell level and we can see that this [structure] makes a difference in the group behaviors of the bacteria, “ said Vernita Gordon, assistant professor of physics in the College of Natural Sciences and corresponding author on the study, published recently in the journal Langmuir.
The technique described by Gordon and her colleagues lets scientists place individual bacteria into any pattern or design they choose and allows for the mingling of multiple species of bacteria on the same surface.
Known as laser, or optical, trapping, the method uses a focused laser beam as a manipulative tool.
The secret to this process is that light refracts, or bends, when it travels from one material to another. Anyone who has observed how a straw in a glass of water looks bent has observed refraction in action.
The laser light is focused onto a single bacterium floating in a nutrient liquid. Since the microbe refracts light differently than does the liquid surrounding it, the light gets bent slightly outward when it encounters the cell. As the light changes direction, it applies an equal and opposite force to the bacterium, pinning the microbe inside the focus of the beam. The bacterium can be moved by simply changing the position of the laser focus with respect to the environment. By lowering the focus onto the surface of the biofilm growth chamber, the bacterium can be placed on the surface with micron-level precision. This method can be used to build up arbitrary configurations of bacteria that grow to form a biofilm with a spatial structure determined by the initial conditions the researchers create.
Gordon and her colleagues were able to demonstrate that laser trapping did not harm the microbes or affect their normal behavior or growth, indicating that the method is a safe and effective way of placing individual bacterium onto a substrate to study the formation of multi-species biofilms.
Joint first authorship on this study is held by Jaime Hutchison, a postdoctoral fellow, and Christopher Rodesney, a Ph.D. student, both from UT Austin. Other authors include Karishma Kaushik, Henry Le, and Daniel Hurwitz from UT Austin and Yasuhiko Irie from the University of Bath. This work was supported by startup funds from UT Austin and by a grant from the Human Frontiers Science Project.
To read the paper “Single-Cell Control of Initial Spatial Structure in Biofilm Development Using Laser Trapping” in Langmuir go to: http://pubs.acs.org/doi/full/10.1021/la500128y



